Dual Nature of Radiation and Matter-Revision Notes
CBSE Class 12 Physics
Revision Notes
Chapter-11
Dual Nature of Radiation and Matter
- Electric Discharge: The passage of an electric current through a gas is called electric discharge.
- Discharge Tube: A hard glass tube along with the necessary arrangement, which is used to study the passage of electric discharge through gases at low pressure, is called a discharge tube.
- Cathode Rays: Cathode rays are the stream of negatively charged particles, electrons which are shot out at a high speed from the cathode of a discharge tube at a pressure below 0.01 mm of Hg.
- Work Function: The minimum amount of energy required by an electron to just escape from the metal surface is known as the work function of the metal.
- Electron Emission: The minimum amount of energy required by an electron to just escape from the metal surface is known as the work function of the metal.
- Thermionic Emission: Here electrons are emitted from the metal surface with the help of thermal energy.
- Field or Cold Cathode Emission: Electrons are emitted from a metal surface by subjecting it to a very high electric field.
- Photoelectric Emission: Electrons emitted from a metal surface with the help of suitable electromagnetic radiation.
- Secondary Emission: Electrons are ejected from a metal surface by striking over its fast-moving electrons.
- Forces Experienced by an Electron in Electric and Magnetic Fields:-
- Electric field: The force FE experienced by an electron e in an electric field of strength (intensity) E is given by, FE = eE
- Magnetic field: The force experienced by an electron e in a magnetic field of strength B weber/m2 is given by, FB = Bev
where v is the velocity with which the electron moves in the electric field and the magnetic field, perpendicular to the direction of motion. - If the magnetic field is parallel to the direction of motion of the electron, then, FB = 0.
- Photoelectric Effect: The phenomenon of emission of electrons from the surface of substances (mainly metals), when exposed to electromagnetic radiations of suitable frequency, is called photoelectric effect and the emitted electrons are called photoelectrons.
- Maximum K. E of the Photoelectrons Emitted from the Metal Surface:
(Einstein’s Photoelectric equation) - Cut Off or Stopping Potential: The value of the retarding potential at which the photoelectric current becomes zero is called cut off or stopping potential for the given frequency of the incident radiation.
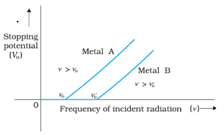
- Threshold Frequency: The minimum value of the frequency of incident radiation below which the photoelectric emission stops altogether is called threshold frequency.
- Laws of Photoelectric Effect:
- For a given metal and radiation of fixed frequency, the number of photoelectrons emitted is proportional to the intensity of incident radiation.
- For every metal, there is a certain minimum frequency below which no photoelectrons are emitted, howsoever high is the intensity of incident radiation. This frequency is called the threshold frequency.
- For the radiation of frequency higher than the threshold frequency, the maximum kinetic energy of the photoelectrons is directly proportional to the frequency of incident radiation and is independent of the intensity of incident radiation.
- The photoelectric emission is an instantaneous process.
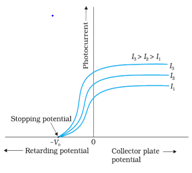
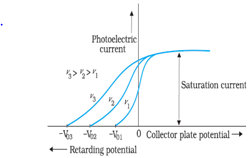
- Einstein’s Theory of Photoelectric Effect:-
- Einstein explained the photoelectric effect with the help of Planck’s quantum theory.
- When radiation of frequency is incident on a metal surface, it is absorbed in the form of discrete packets of energy called quanta or photons.
- A part of the energy of the photon is used in removing the electrons from the metal surface and remaining energy is used in giving kinetic energy to the photoelectron.
- Einstein’s photoelectric equation is,
Where o is the work function of the metal. - If is the threshold frequency, then
- All the experimental observations can be explained on the basis of Einstein’s photoelectric equation.
- Photocell:
- It is an arrangement which converts light energy into electric energy.
- It works on the principle of the photoelectric effect.
- It is used in cinematography for the reproduction of sound.
- Dual Nature of Radiation: Light has dual nature. It manifests itself as a wave in diffraction, interference, polarization, etc., while it shows particle nature in the photoelectric effect, Compton scattering, etc.
- Dual Nature of Matter:
- As there is complete equivalence between matter (mass) and radiation (energy) and the principle of symmetry is always obeyed, de Broglie suggested that moving particles like protons, neutrons, electrons, etc., should be associated with waves known as de Broglie waves and their wavelength is called de Broglie wavelength.
- The de Broglie wavelength of a particle of mass m moving with velocity v is given by-
where h is Planck’s constant.
- De Broglie Wavelength of an Electron: The wavelength associated with an electron beam accelerated through a potential V is given by
in angstrom for electron beam. - Heisenberg Uncertainty Principle:
where is uncertainty in position & is uncertainty in momentum - Electron Microscope:
- It is a device that makes use of accelerated electron beams to study very minute objects like viruses, microbes and the crystal structure of solids.
- It has a magnification of .
- Davisson-Germer Experiment: This experiment help to confirm the existence of de Broglie waves associated with electrons. The arrangement is shown in the figure below:
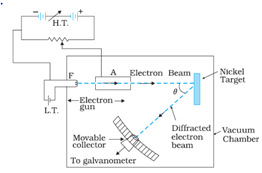
The intensity of the scattered beam of electrons is found to maximum when the angle of scattering is 50o and the accelerating potential is 54V. According to the de-broglie hypothesis,
This proves the existence of de-Broglie waves for slow-moving electrons.